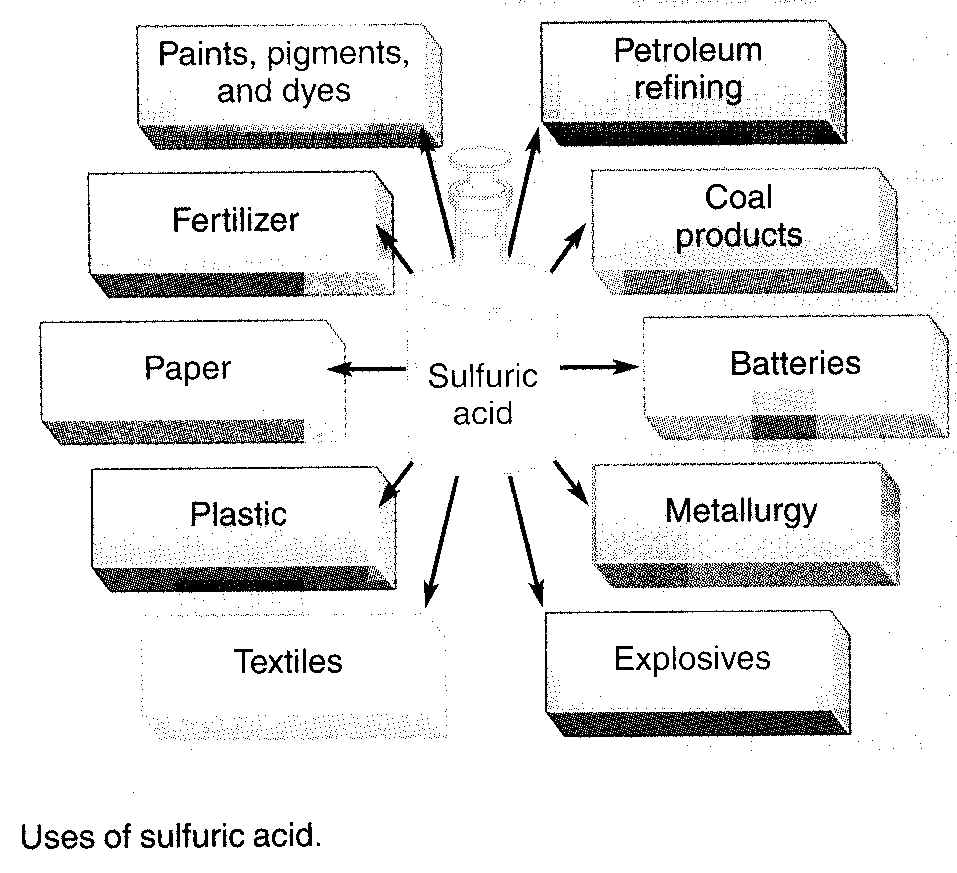
SULFURIC ACID:NUMBER ONE IN INDUSTRY
Sulfuric acid (H2SO4) is the number one Inorganic chemical produced in the world. Millions of tons of this oily, odorless, colorless, and corrosive substance are used each year to manufacture products that range from construction materials to vanilla flavoring. Most sulfuric acid is used to help feed us, because about 70% of the annual production is used to make fertizers.

Sulphuric acid is prepared commercially by burning elemental sulphur to give sulphur dioxide gas:
S(l) + O2(g) ® S02(g)
Some SO2 is also obtained as a by product of other processes such as copper and zinc production. The SO2 from these sources is catalytically reacted with oxygen to produce gaseous sulphur trioxide:
2S02(g) + 02(g) ![]() 2SO3(g)
2SO3(g)
The sulfur trioxide is then dissolved in concentrated sulphuric acid to form oleum which in turn forming desired concentration of sulphuric acid by adding appropriate amount of water:
SO3(g) + H2SO4(l) ® H2S2O7(l)
H2S2O7(l) + H2O(l) ® 2H2SO4(l)
Sulphric acid is used in automobile batteries, in some drain cleaners, and in the manufacturing of numerous consumer products. Although the fertilizer industry uses the most sulphuric acid in the production of phosphate and ammonium sulphate salts, the metal industries also use significant amounts for pickling iron and steel. Pickling involves dipping a metal into sulphuric acid to remove metal oxides from the surface prior to plating the iron or steel with other metals. Practically every major manufacturing industry uses some sulfuric acid.