Accidental Formation of a Diaryl Ether : Dioxin (¤Gäú§¨)
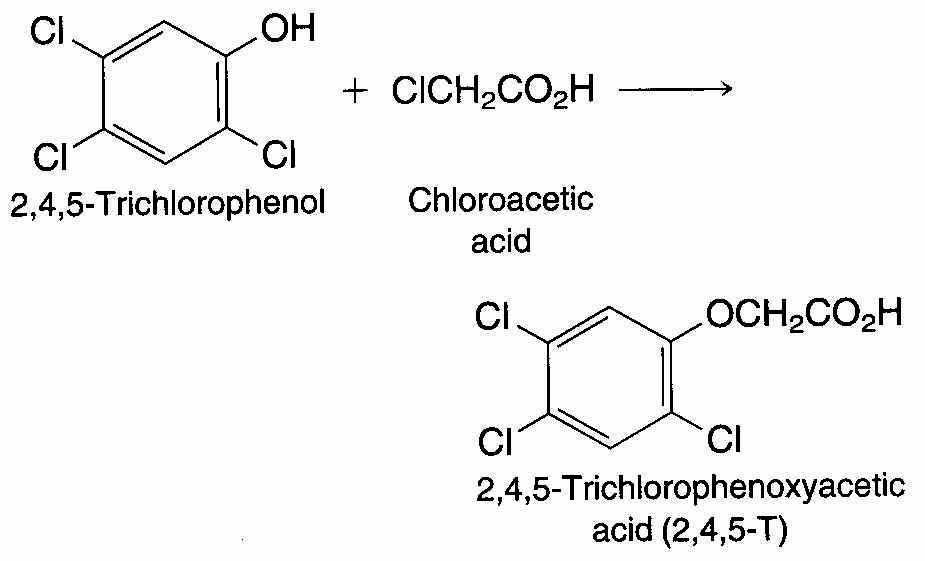
Until its use was regulated in 1979, 2,4,5-T(2,4,5-trichlorophenoxyacetic acid) was a widely used herbicide. It is prepared by reaction of 2,4,5-trichlorophenol with chloroacetic acid.

The starting material for the process, 2,4,5-trichlorophenol, is almost always contaminated with small amounts of the diaryl ether 2,3,7,8-tetrachlorodibenzo-p-dioxin, better known simply as dioxin.
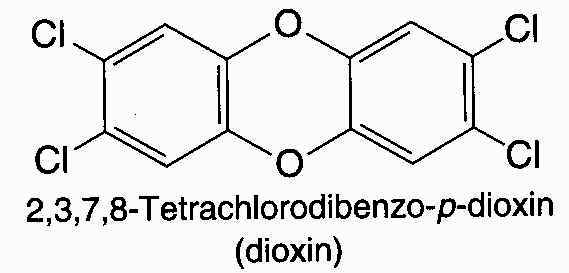
Dioxin is carried along with 2,4,5-trichlorophenol is converted to 2,4,5-T, and it enters the environment when 2,4,5-T is sprayed on vegetation. Typically, the amount of dioxin present in 2,4,5-T is very small. Agent Orange, a 2,4,5-T-based defoliant used during the Vietnam War, contained about 2 ppm of dioxin.
Tests with animals have revealed that dioxin is one of the most toxic substances known. in mice, it is about 2000 times more toxic than strychnine and about 150,000 times more toxic than sodium cyanide. Fortunately, however, available evidence indicates that humans are far more resistant to dioxin than are test animals, and so far there have been no fatalities ddirectly attributablt to dioxin. The most prominent symptom seen so far has been a severe skin disorder known as chloracne. Yet to be determined are the long-term effects of dioxin exposure. A connection between exposure to dioxin and the occurrence of a rare type of cancer has been suggested on the basis of a statistical study.
There is another environmental source of dioxin -- polychlorinated biphenyls, or PCBs(¦h´âÁpf).
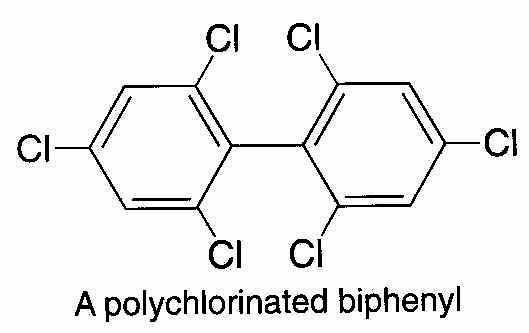
When PCBs are strongly heated as during a fire, one of the decomposition products is dioxin. As a result, firefighters take extra precautions when fighting electrical or other fires in which PCB-containing fluids might be present.