Lactose Intolerance and Galactosemia
Lactose is the principal carbohydrate in milk. Human mother's milk obtained by nursing infants contains 7% - 8% lactose, almost double the 4% - 5% lactose found in cow's milk.
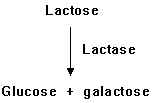
For many people, the digestion and absorption of lactose is a problem. This problem, called lactose intolerance, is a genetic condition in which people lack the enzyme lactase, which is needed to hydrolyse lactose to galactose and glucose.

Deficiency of lactase can be caused by a genetic defect, by physiological decline with age, or by injuries to the mucosa lining of the intestines. When lactose molecules remain in the intestine undigested, they attract water to themselves, causing fullness, discomfort, cramping, nausea, and diarrhea. Bacterial fermentation of the lactose further along the intestinal tract produces acid (lactic acid), adding to the discomfort.
The level of the enzyme lactase in humans varies with age. Most children have sufficient lactase during the early years of their diet. In adulthood, the enzyme level decreases, and lactose intolerance develops. This explains the change in milk-drinking habits of many adults. Some experts estimate that as many as one of three adult Americans exhibits a degree of lactose intolerance.
After lactose has been degraded into glucose and galactose, the galactose has to be converted into glucose before it can be used by cells. In humans, the genetic condition called galactosemia is caused by the absence of one or more of the enzymes needed for this conversion. In people with this condition, galactose and its toxic metabolic derivative dulcitol accumulate in the blood.
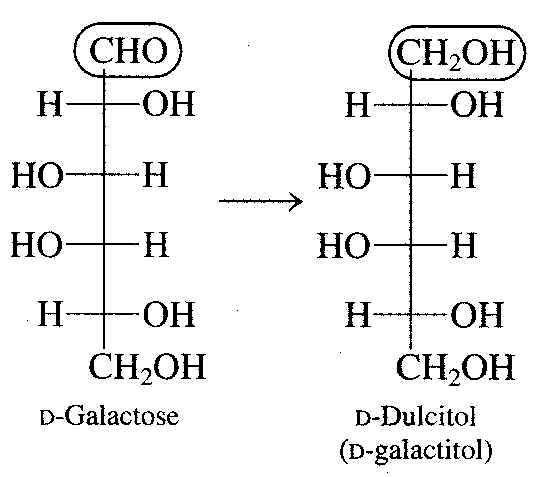
If not treated, galactosemia can cause mental retardation in infants and even death. Treatment involves exclusion of milk and milk products from the diet.