
Buffering Action in Human Blood
Carbonate,phosphate, and protein buffer systems play an important role in maintaining proper pH. Even small departures from blood's normal pH range of 7.35 - 7.45 can cause serious illness, and death can result from pH variations that exceed a few tenths of a unit. Many of the key reactions that occur in blood are enzyme-catalysed and reach optimum conditions only within blood's normal pH range. Outside this range, enzyme action slows down or even stops.
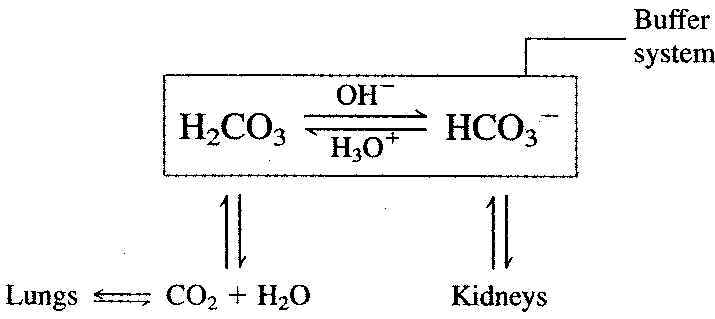
The carbonate buffer system involves the species of H2CO3 and HCO3°¬. Any excess acid formed in the blood reacts with the HCO3°¬ ion, and any excess base reacts with H2CO3.

The ratio of [H2CO3] to [HCO3°¬] in blood is approximately 1 to 10, which means this buffer has a greater ability to interact with acid than with base. Significant amounts of acids (up to 10 moles a day) are produced in human body as a result of normal metabolic reactions. For example, lactic acid is produced in muscle tissue during exercise.
This 1-to-10 ratio of buffering species is also needed to maintain the blood at a pH of 7.4. A 1-to-1 ratio buffer would produce a pH of 6.4. The 1-to-10 ratio is easily maintained. Excess H2CO3 decomposes to CO2 and H2O and is removed from the blood by the lungs.
![]()
Excess HCO3°¬ can be eliminated from the body through the kidneys.
The phosphate buffer system involves the species dihydrogen phosphate (H2PH4°¬) and hydrogen phosphate (HPH4°¬). The control of pH using this system occurs by OH°¬ ion reacting with H2PO4°¬ and by H3O+ reacting with HPO4°¬.
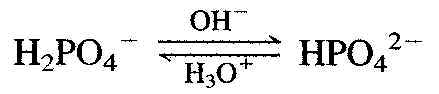
A 3-to-5 ratio of [H2PO4°¬] to [HPO4°¬] is required to maintain p pH of 7.4.
Under certain stress conditions, the blood's buffer system can be overwhelmed. Acidosis is a body condition in which the pH of blood drops from its normal value of 7.4 to 7.1-7.2. Alkalosis is a body condition in which the pH of blood increases from its normal value of 7.4 to a value of 7.5. Both can be life-threatening if not properly taken care of; both can be caused by either metabolic processes or changes in breathing patterns (respiration).
Metabolic acidosis is seen in diabetics, who accumulate acidic substances from the metabolism of fats. Excessive loss of bicarbonate ion in cases of severe diarrhea is another cause. A temporary metabolic acidosis condition can result from prolonged intensive exercise. Exercise generates lactic acid (a weak acid) in the muscles. Some of the lactic acid ionizes, and this produces an influx of H3O+ ions into the bloodstream.
Metabolic alkalosis is less common than metabolic acidosis. It results from elevated HCO3°¬ ion levels. Causes include prolonged vomiting and the side effects of certain drugs that change the concentrations of sodium, potassium, and chloride ions in the blood.
Respiratory acidosis results from higher that normal levels of CO2 in the blood; inefficient CO2 removal is usually the origin of this problem. Hypoventilation (a lowered breathing rate), caused by lung diseases such as emphysema and asthma or obstructed air passages, produces respiratory acidosis.
Respiratory alkalosis is caused by hyperventilation (an elevated breathing rate). Causes include hysteria and anxiety (caused by chemistry tests --jokes!) and the rapid breathing associated with extremely high fevers.