Electrons in Excited States
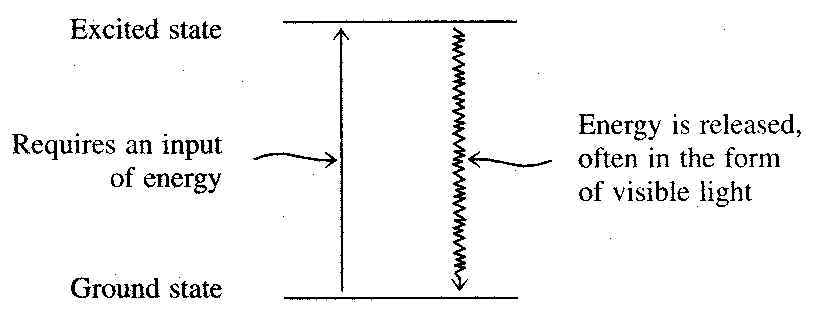
When an atom has its electrons positioned in the lowest-energy orbitals available, it is said to be in its ground state. An atom's ground state is the normal (most stable) state for the atom.
It is possible to elevate electrons in an atom to high-energy unoccupied orbitals by subjecting the atom to a beam of light energy, an electrical discharge, or an influx of heat energy. With electrons in higher, normally unoccupied orbitals, the atom is said to be in an excited state. An excited state is an unstable state that has a short life span. Quickly, the excited electrons drop back down to their previous positions (the ground state). Accompanying the transition from excited state to ground state is a release of energy. Often this release of energy is in the form of visible light.

The principle of electron excitation through input of energy has been found to have useful applications in many areas.
1. "Neon" Advertising Signs. In such signs, gaseous atoms are excited by an electric discharge to produce a variety of colours; the colour depends on the identity of the gas. Neon gas produces an orange-red light, argon gas a blue-purple light, and krypton gas a white light.
2. Street and Highway Lights. Such lights involve energy emitted by electrically excited metal atoms. Mercury vapour lamps produce a yellow light that can penetrate fog farther than does light from a sodium vapour lamp. On the other hand, sodium vapour lamps are more energy-efficient in their operation.
3. Fireworks. Metals atoms excited by heat are responsible for the colour of fireworks. Strontium (red colour), barium (green colour), copper (blue colour), and aluminium (white colour) are some of the metals involved. The metals are present in the fireworks in the form of metal-containing compounds rather than as pure metals.

The different colours of fireworks result when heat excites the electrons of different kinds of metal atoms present.
4. Identification of Elements. Each of the known elements, when electrically excited in the gaseous state, produces a unique pattern of wavelengths of emitted light (radiation). This emission pattern, which is called an atomic spectrum, serves as a "fingerprint" for the element and can be used to distinguish the element from any other.
5. Analysis of Human Blood Fluids. Instruments called atomic spectrometers are now used to analyse body fluids for the presence of particular elements (in free or combined form). It is possible to determine concentrations of species with such instruments, which are now found in almost all clinical chemistry laboratories. The concentration of sodium and potassium in a particular fluid can be obtained from the intensity of light emitted by excited atoms of these elements. Atomic spectroscopy makes it possible to measure the amount of lead in a patient's blood or urine (in cases of lead poisoning) by using a sample as small as 0.01 cm3.