Hair Care and pH -- A Delicate Balance
Hair shampoo advertisements often proclaim the proper pH for their products, but does controlling the pH of hair care products really make hair cleaner, shiny, or stronger?

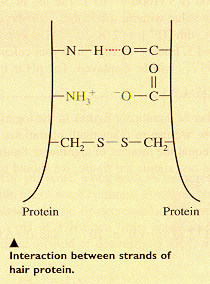
Each strand of hair is composed of many long chains of amino acid linked together as polymers called proteins. The individual chains can connect with other chains in one of three ways: (1) hydrogen bonds, red; (2) salt bridges (the result of acid-base interactions), green; and (3) disulfide bonds, blue. These interactions are shown in the diagram.
When hair is wet with water, the hydrogen bonds are broken. As the wet
hair is shaped, set, or dried, the hydrogen bonds form at new positions and hold the hair
in the style desired. If an acidic solution (pH 1.0-2.0) is used on the hair, the hydrogen
bonds and the salt bridges are both broken, leaving only the disulfide bonds to hold the
chains together. In a mildly alkaline solution (pH 8.5) some of the disulfide bonds are
also broken. The outer surface of the hair becomes rough, and light
does not reflect evenly from the surface, making the hair look dull. Using an
alkaline shampoo will cause damage by continued breakage of the disulfide bonds, which
results in "split ends." If the pH is increased
further to approximately 12.0 the hair dissolves as all types of bonds break. This is the
working basis for depilatories (hair removers), such as Neet![]() and Nair
and Nair![]() .
.

Hair has its maximum strength at pH 4.0-5.0. Shampooing tends to leave the hair slightly alkaline, so an acid rinse is sometimes used to bring the pH back into the normal range. Lemon juice or vinegar are common household products that are used for this purpose. The shampoo may also be "acid-balanced," containing a weak acid (such as citric acid) to counter-act the alkalinity of the solution formed when the detergent interacts with water.